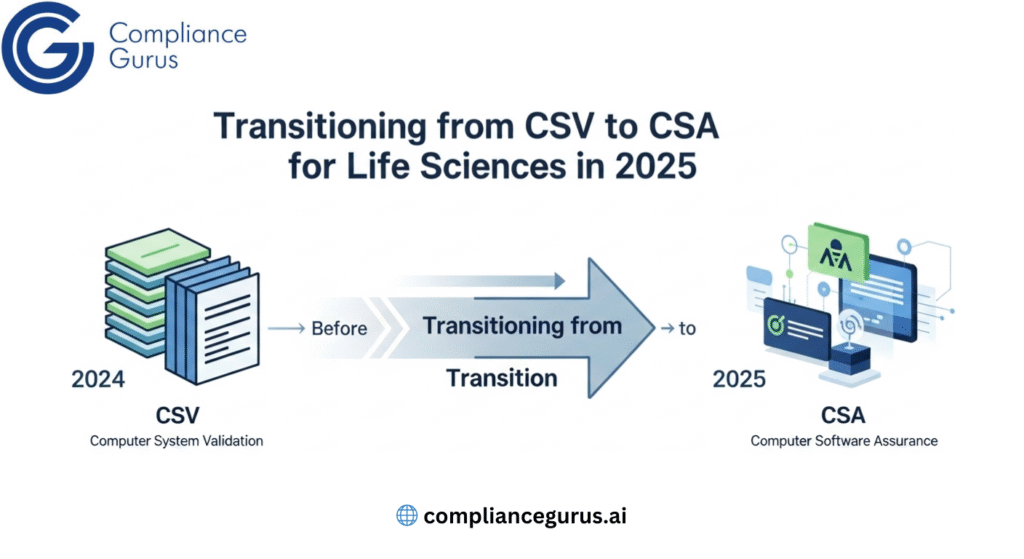
Let’s be honest that staying on top of all the rules in life sciences can get stressful fast. Maybe you’ve stared at FDA documents, or heard about something called Annex 11, and thought, “How does this fit into my day-to-day job?” If that’s you, you’re not alone. Most people in labs and production lines aren’t lawyers. You want clear answers, simple plans, and real results.
That’s exactly why this guide exists. Think of it as a roadmap that is easy to follow, rooted in daily work, and built to help you get from confusion to clarity. We’ll walk through what matters about 21 CFR Part 11, Annex 11, and ALCOA+, and how trusted experts can turn all the big words into small, actionable steps that work for your team.
Getting to Grips with the Big Three: 21 CFR Part 11, Annex 11, ALCOA+
When people talk compliance, these three terms come up again and again. What do they actually mean for you?
- 21 CFR Part 11

This regulation is about how electronic records and signatures are managed. The FDA wants to be sure that digital records are just as reliable as paper ones. That covers everything from how you collect data, to how you protect and store it, to how you show it’s authentic. If data runs through a computer, 21 CFR Part 11 probably applies.
- Annex 11
Annex 11 comes from the European Union. It asks similar questions, but from a slightly different angle. If you sell or want to sell into Europe, you need to pay attention. It looks closely at how you validate software and computerized systems, making sure everything runs honestly, securely, and in a way, regulators can trust.
- ALCOA+ Principles in Software Validation
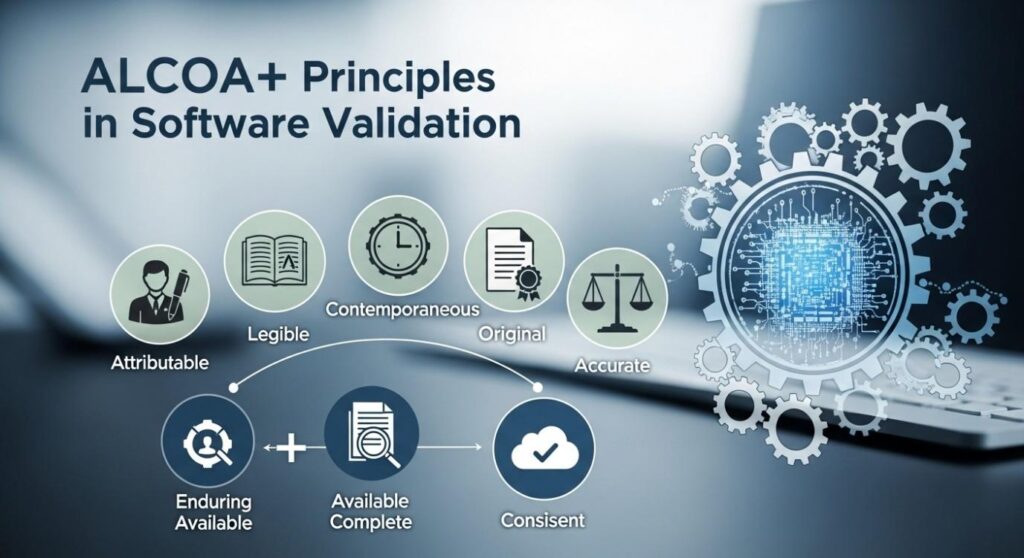
ALCOA+ simplifies how to think about data integrity. Each letter stands for something important:
- Attributable: Who did what, when?
- Legible: Can you read and understand it?
- Contemporaneous: Recorded at the time, not later.
- Original: This is the true record, not a copy.
- Accurate: It’s correct, no mistakes.
The “+” adds a few more keys: Complete, Consistent, Enduring, and Available. These aren’t just buzzwords – they’re tools. They help you avoid mistakes, prove your point in an audit, and keep trust strong.
Why Do So Many Teams Struggle with This?
If compliance feels hard, you haven’t done anything wrong. Life science rules are thick. They change. Resources are tight. People get busy. And the language? Not exactly what you’d chat about in the breakroom.
Here’s why it trips folks up:
- Most teams aren’t full of compliance experts.
- Changes to software or processes can bring new risks.
- Staff are focused on science or production, not endless documentation.
You’re working hard. No one wants to spend their day guessing at what an inspector might ask months from now.
How Consulting and Validation Firms Bridge the Gap?
That’s where a real FDA compliance consulting firm comes into play. Imagine having a smart, experienced friend who’s seen hundreds of audits, fixed roadblocks for all kinds of companies, and actually explains things clearly. That’s what expert consulting does – it gives you backup you can count on.
What Do These Experts Actually Do?
- Translate technical guidance into simple, daily routines.
- Fix what’s missing and lay out a plan, from the first assessment to the last check.
- Build living systems, not just binders for the bookshelf.
- Apply ALCOA+ principles in software validation so your data is solid.
Turning Assessment into Real, Actionable Solutions
So, what’s the process of assessment to actionable solutions, really? It’s not magic. It’s practical, step-by-step, and hands-on.

- Assessment
Consultants start by listening. They check your current processes, software, and records against FDA rules and EU guidelines. No guesswork, just facts.
- Clear Plans
You get a map, not a maze. Every action is spelled out: what the rule says, what you need, what can wait.
- Delivery
Whether it’s new software, fresh training, or rebuilding an audit trail, your team gets practical support. Every move gets you closer to safe, compliant, documented processes.
- Review
Validation is ongoing. Experts show up for regular check-ins and teach your team how to keep the ball rolling – no forgotten steps, no scrambling before audits.
Concrete Services You Can Expect
- FDA Remediation That Sticks
Got a warning letter or 483 inspection findings? Fast, effective remediation is a must. You don’t want patches – you want real answers. Experts get to the root of the issue and build fixes that prevent repeat problems, not short-term plugs.
- Compliance Audits, Minus the Stress
Audits shouldn’t feel like a pop quiz. With help, you’ll be ready every time, not just hoping for the best. Consulting teams handle vendor and in-house audits with the detail and structure inspectors expect, using both 21 CFR Part 11 and Annex 11 software validation.
- Built-In Data Integrity
ALCOA+ isn’t extra paperwork. It makes records reliable – everywhere, always. Consultants help you design systems around these principles so your records hold up, whether you’re dealing with a busy lab or a global supply chain.
Benefits You’ll Notice (And Your Team Will Thank You For)
- Personalized Support: No templates. Your team, your risks, your solutions.
- Simpler Processes: Get documentation and validation that are actually understandable.
- Less Risk: Avoid fines, audit failures, or missed business deals.
- Coaching, Not Just Consulting: Teams don’t just “pass the test.” They learn why, how, and what to do next time.
Tips for Life Science Teams Right Now
- Don’t lean on vendor templates as the answer – they’re a start, not the finish.
- Keep ALCOA+ in daily habits, not just big projects.
- Make sure your records are always clear, organized, and ready to review.
The Smartest Move? Get Trusted Support
The life sciences world never stands still. Rules change. Technology shifts. Markets move. If you want compliance that doesn’t just keep up but leads, you can’t do it alone. The best experts translate every bit of FDA software validation guidance into steps anyone can follow.

Why take the hard route? Choose the easier, safer path – one where regulations aren’t a hassle, but a source of strength and confidence.
Ready to Begin? Here’s Your Next Step
At Compliance Gurus, making complicated guidance simple is in our DNA. We don’t do cookie-cutter. We figure out your needs, create assessments for actionable solutions, and walk you through every stage until the audit feels like just another meeting, not a nightmare.
If you’re serious about mastering compliance and protecting your work, your team, and your business, now’s the moment. Reach out for a pressure-free conversation and turn compliance into your competitive edge.
Key points to remember:
Ready to feel confident, clear, and audit-ready? Compliance Gurus can help you cross every “T” and dot every “I” – with less stress, more support, and zero wasted time.