When data is right, work flows right. Strong data practices cut noise, speed decisions, and keep inspectors satisfied. In regulated life sciences, that’s not a “nice to have” – it’s daily survival. Robust, site-wide data integrity programs and assessments, backed by smart software assurance and disciplined auditing, turn compliance from a scramble into a steady habit that boosts efficiency across labs, plants, and platforms.
Why Data Integrity Is the Engine of Both Efficiency and Compliance?
The mature data integrity programs and assessments protect the full data lifecycle – how data is collected, processed, stored, and managed – so teams trust results and move faster with less rework. It’s built on three pillars that regulators also care about: patient safety, product quality, and regulatory compliance. Done well, it scales from a single site to global operations and is designed to hold up under audit or inspection at any time.
What this looks like in practice:
- Tailored frameworks that keep data accurate, secure, and compliant with FDA, EMA, and other global requirements.
- Thorough assessments to find gaps in systems, processes, and documentation before they become findings.
- Actionable recommendations for executives that improve control and transparency, not just paperwork.
- Implementation support that hardens controls so the program consistently withstands regulatory scrutiny.
The outcome: a rock-solid integrity baseline that protects patient safety, supports product quality, and keeps organizations inspection-ready while reducing waste and delay across teams.
From Assessment to Action: A Clear Roadmap That Works
Strong programs begin with a focused assessment and gap analysis. Experts review how data moves through tools and workflows, identify weak points, and then deliver practical, prioritized fixes that match FDA expectations. After leadership approval, changes are implemented and embedded so controls become a daily routine, not a one-off clean-up.
Typical steps include:
- Evaluate collection, processing, storage, and management practices for alignment with regulatory standards.
- Identify gaps that can risk compliance, such as access control, audit trails, documentation discipline, and training, which are common hotspots.
- Provide tailored recommendations to close risks with durable processes and technical controls.
- Implement and stabilize improvements so the program stands up to audits and inspections on demand.
This “assess – recommend – implement” cycle is simple, proven, and repeatable across sites and systems.
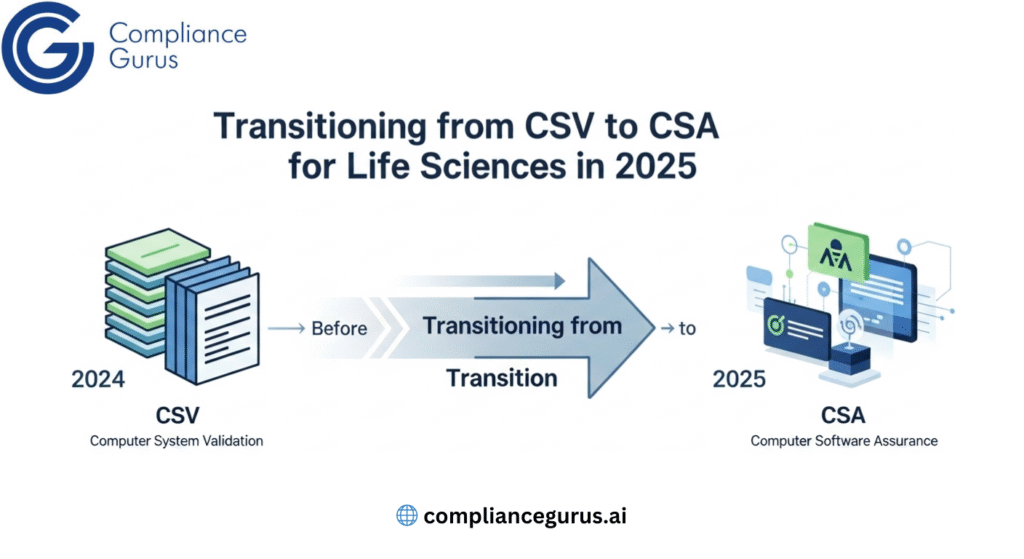
Pair with Risk-Based CSA and Proven CSV to Reduce Burden and Speed Updates
In regulated environments, computer system validation (CSV) and computer software assurance (CSA) are core to keeping GxP applications compliant and dependable. CSV confirms systems perform as intended with the required documentation and testing; cloud-based software assurance applies a risk-based lens so effort goes where it matters most, cutting unnecessary paperwork and keeping systems current.
Key points:
- CSV verifies fitness for intended use across the lifecycle with specifications, protocols, and monitoring.
- CSA focuses testing on the riskiest functions, using vendor evidence where appropriate to accelerate validation without weakening control.
- Both support 21 CFR Part 11 and global standards while enhancing data integrity and patient safety.
- Periodic review ensures GxP applications remain in a validated state over time.
This balanced approach reduces validation drag, helps teams release changes faster, and strengthens inspection readiness simultaneously.
Don’t Skip the Audit: Targeted Audits Prevent Expensive Surprises
Software vendor audits and validation package audits are not optional in FDA-regulated work – they are due diligence that protects the business before selection, validation, and implementation. Many teams buy before auditing; it’s still possible to audit pre-implementation, but auditing prior to selection is strongly advised to confirm 21 CFR Part 11 compliance and reveal the true cost of ownership if configuration or customization is needed.
What effective audits deliver:
- Independent review of vendor capability and product alignment with Part 11 expectations.
- Validation package audits (CSV/CSA) that confirm completeness and an ongoing validated state.
- Practical guidance on avoiding vendor validation templates that often lack Part 11 rigor and require rework.
- Periodic reviews to maintain validation status for the life of the system, especially useful for teams without in-house CSV/CSA SMEs.
Audits are the fastest way to lower risk, keep projects on schedule, and avoid remediation later.
When FDA Attention Turns Your Way: Respond Fast and Fix Precisely
If an Untitled Letter, 483, Warning Letter, or Consent Decree lands, time and precision matter. The right response pairs speed with a clear plan that addresses root causes, prioritizes fixes, and documents restored control.
Effective steps include:
- Rapid review of FDA findings to build a compliant remediation plan and timeline.
- Assessment of current Data Integrity, CSV, and 21 CFR Part 11 practices to identify exact gaps.
- Implementation support to execute remediation and return systems to an FDA-compliant state.
Done right, FDA remediation not only resolves the citation – it leaves processes stronger and inspection-ready for the next review.
How to Start: A Simple, Durable Plan?
- Begin with targeted Data Integrity Services and Assessments to map gaps in systems, processes, and records across the data lifecycle.
- Prioritize fixes that protect patient safety, product quality, and compliance first; align with 21 CFR Part 11 where applicable.
- Implement risk-based CSA alongside CSV to focus effort on what matters most while maintaining compliance.
- Audit software vendors before selection and review validation packages periodically to sustain a validated state.
- If cited, respond quickly with a structured FDA remediation plan that addresses root causes and restores control.
This path builds resilience while reducing operational friction.
A Quick Checklist for Leaders
- Are data controls mapped to safety, quality, and regulations, and do they operate consistently across sites?
- Is there a current gap analysis with executive-level, actionable recommendations, and have changes been implemented?
- Do validation strategies blend CSV with risk-based CSA to avoid over-testing while meeting Part 11?
- Were vendors audited before selection, and are validation packages reviewed periodically?
- If an FDA letter arrived tomorrow, could the team produce a timely, compliant plan with evidence of restored control?
If any answer is “not sure,” start with an assessment and move forward from there.
The Bottom Line
Efficiency and compliance do not compete in biotech – they reinforce each other when built on a solid data integrity foundation. With site-wide programs, risk-based CSA/CSV, focused audits, and disciplined remediation, teams create a system that runs smoothly day to day and stays ready for inspections any day.
Ready to Lower Risk and Move Faster?
Compliance Gurus supports the full journey from designing and implementing data integrity programs and assessments to risk-based CSA/CSV, vendor and validation audits, and time-critical remediation planning and execution. For a practical starting point, begin with an assessment and turn insights into durable controls that scale.