A plan is not just a list. It’s what guides everyday work and makes sure small details don’t become big problems.
In almost every workplace today, technology is part of daily routines. From scheduling to setting up labs, computer systems keep things running smoothly for everyone. When these tools are used in places like medicine or science, making sure they work right is important for safety and trust. Creating a proper Validation Master Plan, especially for CSV, helps each step go as it should and keeps surprises away.
This guide shares exactly what goes into a good validation plan, explains the process in everyday terms, and tells you how some companies slip up so you can avoid those mistakes.
What Is a Validation Master Plan CSV?
A Validation Master Plan (VMP) is like an easy-to-follow recipe. It lays out all the steps needed for getting, setting up, and running computer systems in places where rules and safety matter. In companies that deal with pharmaceuticals, drugs, medical testing, or biotechnology, the plan shows how each part of the system should be checked, tested, and watched closely so that everyone, like managers, workers, and auditors, knows the system does its job safely.

Having this plan means everyone knows where they stand, who does what, and how things are double-checked. The idea is to make validation lifecycle management simple and routine. Instead of trying to memorize a bunch of steps, the plan tells you what’s important and when to check things.
Why Do Regulated Industries Need a VMP?
- A strong validation master plan CSV sets every step-in order, from the earliest check to the last sign-off.
- It saves workers and managers time and avoids confusion. Everyone sees what’s next and what’s already finished.
- The plan also proves to inspectors, auditors, and partners that systems keep running reliably and safely.
- For companies using cloud solutions or validation of SaaS in regulated industries, a VMP makes sure that even outside software stays in line with regulations and does what it promises.
Adding Compliance to System Development Life Cycle SDLC
Good validation means starting with a clear routine, or what is known as the system development life cycle SDLC compliance. This routine is basically breaking the journey of every computer system into easy-to-understand stages:
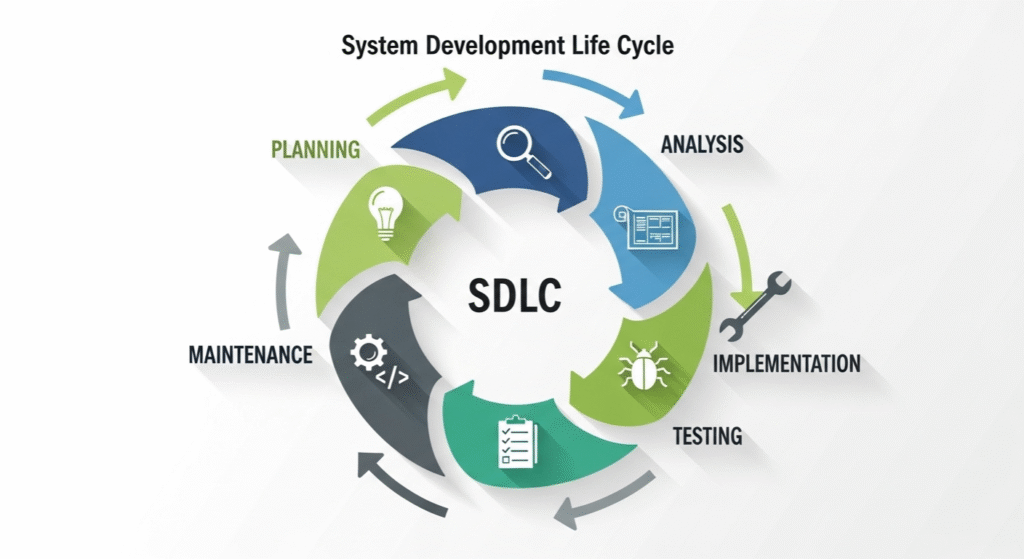
- Planning
Set goals early and decide what rules need to be followed.
- Building
Create and set up the system with care, making notes of what needs testing.
- Trying Out
Carefully test every part and keep records.
- Using
Once the system is active, make sure to check it from time to time for any hiccups or needed improvements.
- Wrapping Up
When a system is retired, safely close everything out and store records for future needs.
Following these steps keeps things clear and helps workers know where their work fits into the big picture.
How to Build a Strong Validation Master Plan CSV?
1. Decide What’s Included Upfront
Imagine you are helping to cook a family meal. You wouldn’t just guess what to buy or cook – you’d make a list, set steps, and maybe even ask others what they want to eat or help with. Building a VMP works in a similar way.
Think about which systems, tools, or sites need attention. Drawing the boundaries early means no one gets surprised later by extra tasks or unclear responsibilities.
2. Map Out the Rules
Every industry has its own set of rules, from FDA 21 CFR Part 11, EU GMP guidelines, to ISO. Look up everything that could affect your plan and jot it down in one place. That way, you’re not trying to follow five sets of instructions at once but can check each part off as you go.
3. Get the Right Mix of People
Don’t let just one department handle it alone. Bring in quality teams, engineers, IT people, and sometimes scientists. Mixing up backgrounds means fewer missed steps, and more people can help spot problems before they happen.
4. Use a Risk-Based Approach
Focus most energy where there is a chance for problems. Not every detail matters the same. For example, patient data might need tighter checks than scheduling software. Use tools like risk ratings to make smart priorities.
5. List Out Documents and Records
Keep everything written in easy language – who tested what, what was found, and what got fixed. Some common papers and files include:
- User Requirements Specification (URS)
- System Designs and Features
- Validation protocols IQ OQ PQ
- Test Scripts showing step-by-step tests while attaching objective evidence
- Change notes
- SOPs and training guides
All of this helps managers and inspectors easily see what was done and why.
Understanding Validation Protocols: IQ OQ PQ

Every time a new computer system is set up, teams use three main checks:
- Installation Qualification (IQ): Check if everything from hardware to software is installed as required. Did it get set up right? Are the wires and programs ready to work?
- Operational Qualification (OQ): Test the system with normal activities. Does it work as it should? Are there any unusual errors or breakdowns?
- Performance Qualification (PQ): Try the system out “in the real world.” Will it keep working smoothly day after day, week after week?
Validation Lifecycle Management: Staying Ready Every Day
Good validation lifecycle management means the job isn’t over once the system is running. Teams have to keep an eye on system performance, do periodic tests, and always update paperwork if anything changes. Simple checks, like watching for system alerts or planning new tests after updates, ensure that software keeps working like promised.
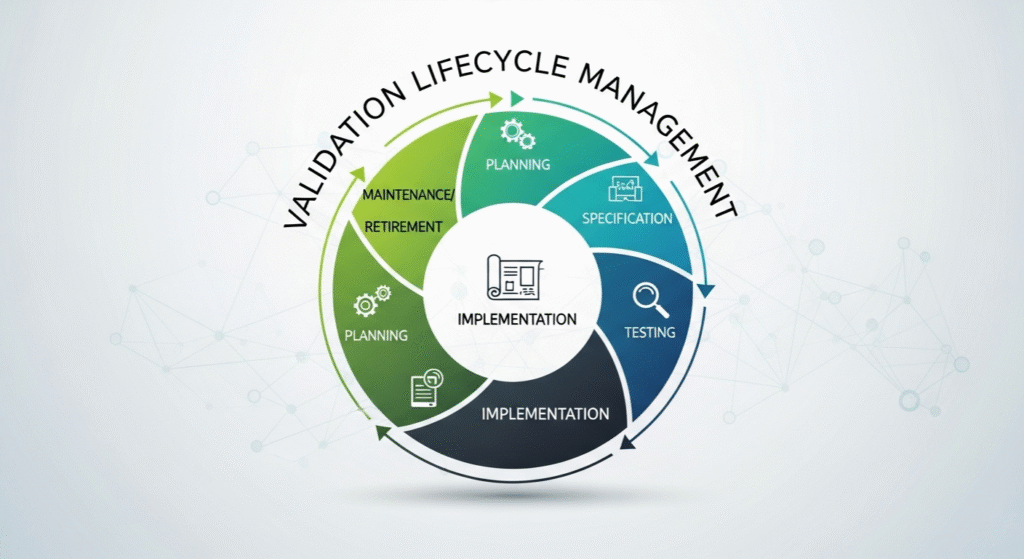
Over time, this routine doesn’t just protect customers – it protects your whole business from unexpected surprises or failed audits.
Common Mistakes and How to Avoid Them
It’s natural for busy teams to slip up now and then. But knowing about common mistakes helps everyone avoid them before they become real problems:
1. Poor or Confusing Documents
- Mistake: Not writing down what was tested or leaving details vague makes it hard for future teams (or inspectors) to understand.
- Tip: Write simply. Use short, clear sentences. Stick to facts and avoid jargon.
2. Getting Lost in the Details
- Mistake: Making records too complicated or adding unnecessary words makes it tough to find what’s essential.
- Tip: Organize notes and files with bullet points and lists. That way, anyone can review them without getting confused.
3. Skipping Planning Steps
- Mistake: Jumping straight into testing or daily use without careful planning means important requirements might be forgotten.
- Tip: Pause and plan well. Assign each task, check along the way, and adjust plans if things change.
4. Isolating Teams
- Mistake: IT or engineering working alone can miss others’ needs.
- Tip: Share decisions and progress openly across teams. Bring in experts from different backgrounds.
5. Forgetting Change Management
- Mistake: Not reviewing or updating validation when systems change.
- Tip: Build scheduled reviews and update paperwork anytime the system is upgraded or procedures change.
Everyday Best Practices
- Start simple with clear steps and easy explanations.
- Focus effort where it matters most, especially for safety and compliance.
- Use training to keep everyone informed of rules and routines.
- Automate simple checks whenever possible to save time and avoid manual mistakes.
Final Thoughts: Turning Compliance into Confidence
Having a clear validation master plan CSV is more than just an “extra task” on a checklist. It helps companies keep work running smoothly, protects safety, and proves that every part of the process can be trusted – day after day, audit after audit.
If creating or updating a CSV or CSA plan feels confusing, the expert team at Compliance Gurus is here to help. Our experience makes it easy for clients to build systems that stand up to every test and keep customers safe. Get in touch with Compliance Gurus and see how simple the compliance process can be – your team deserves the confidence that comes with thorough, reliable validation.