In the life sciences industry, keeping up with rules like 21 CFR Part 11 can feel tough for any of us nowadays. If you are running a small pharmaceutical startup or a big biotech company, making sure your data and systems meet standards is a must. It’s not only about dodging fines or passing inspections. It’s about keeping patients safe, ensuring products are top-notch, and earning trust from regulators and clients.
That’s where data integrity services, Computer System Validation (CSV), and GAMP 5 guidelines step in. These tools build a solid compliance plan, helping your organization stay on track while smoothing out operations.
Why 21 CFR Part 11 Matters for Your Organization?
If you’re in pharmaceuticals, biotech, medical devices, or even food and cosmetics, you’ve likely heard of 21 CFR Part 11. This FDA rule sets standards for handling electronic records and signatures, making sure they’re trustworthy, reliable, and equal to paper records. It’s the backbone for keeping your data and systems safe, accurate, and audit-ready.

Here’s why it’s a big deal:
- Patient Safety
Solid data ensures products meet quality standards, keeping people safe.
- Regulatory Trust
Following rules builds confidence with agencies like the FDA, EU, or MHRA.
- Smooth Operations
Tested systems cut down on mistakes and keep things running well.
The Role of Data Integrity Services in Compliance
Data runs everything in life sciences. From clinical trial results to manufacturing records, every detail needs to be spot-on, secure, and easy to trace. That’s what data integrity services are all about. They help you set up a system that keeps your data safe and meets global rules, including 21 CFR Part 11.

What Are Data Integrity Services?
In plain words, data integrity services mean checking and improving how your company handles data, from collecting it to storing it. It’s like a checkup for your data systems, finding weak spots before they turn into big issues.
Here’s how they help:
- Gap Checks: Experts dig into your systems, processes, and records to spot risks.
- Custom Plans: Solutions fit your company’s size, budget, and goals.
- Global Rules: Programs match FDA, EMA, and other standards.
- Audit Prep: A strong data setup keeps you ready for inspections anytime.
Say a drug company finds their data collection misses proper audit trails. Data integrity services would catch this, suggest fixes like better records or system updates, and set up a plan that passes regulatory reviews. The payoff? Data you can count on, and regulators can trust.
Mastering Computer System Validation (CSV)
If 21 CFR Part 11 is the playbook, Computer System Validation (CSV) is how you make sure your systems play by it. CSV tests and confirms that your computer systems, used in manufacturing or quality control, work right and meet regulatory needs.

Why CSV Counts?
In life sciences, GxP software (used in manufacturing, clinical, or lab work) needs testing to perform as expected. Without it, you risk data mix-ups, system crashes, or failing 21 CFR Part 11. For example, if your software doesn’t control access well, someone could accidentally mess up your data.
Here’s what CSV covers:
- System Plans: Defining how the system should work for users and rules.
- Testing Steps: Deep checks to make sure it runs as planned.
- Constant Checks: Regular reviews to keep the system in top shape over time.
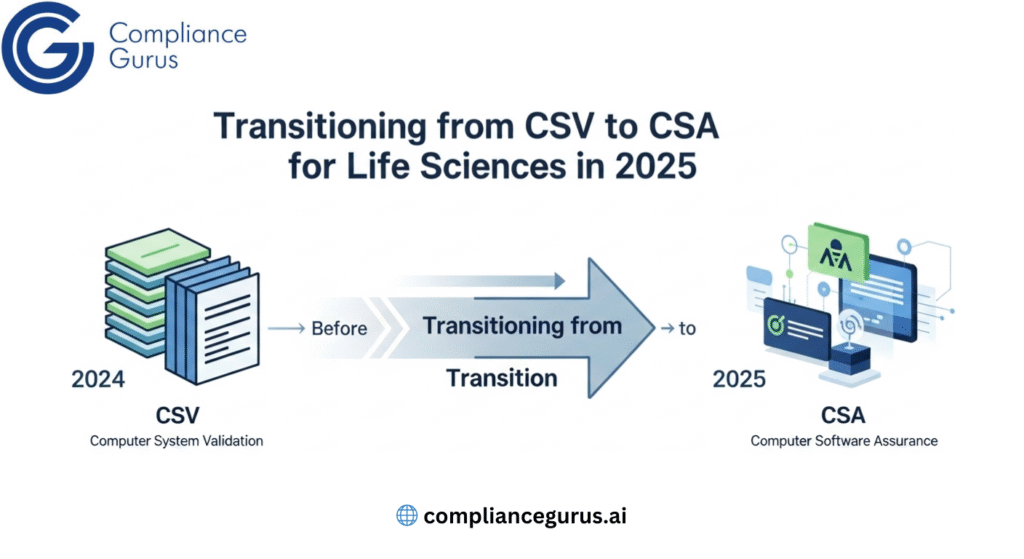
The CSA Option
Lately, the FDA rolled out Computer Software Assurance (CSA), a smarter take on CSV. It focuses on risks, prioritizing patient safety, product quality, and data integrity while cutting extra paperwork. Think of it as a leaner CSV, still nailing compliance with less fuss.

For instance, a biotech company setting up new GxP software might use CSA to focus on risky areas, saving time and money while staying compliant. CSV or CSA, the goal is clear: systems you can rely on that meet 21 CFR Part 11.
Leveraging GAMP 5 Guidelines for Success
The GAMP 5 guidelines (Good Automated Manufacturing Practice) give you a clear plan for validating automated systems in life sciences. Created by the International Society for Pharmaceutical Engineering (ISPE), they’re the go-to standard for meeting rules like 21 CFR Part 11.
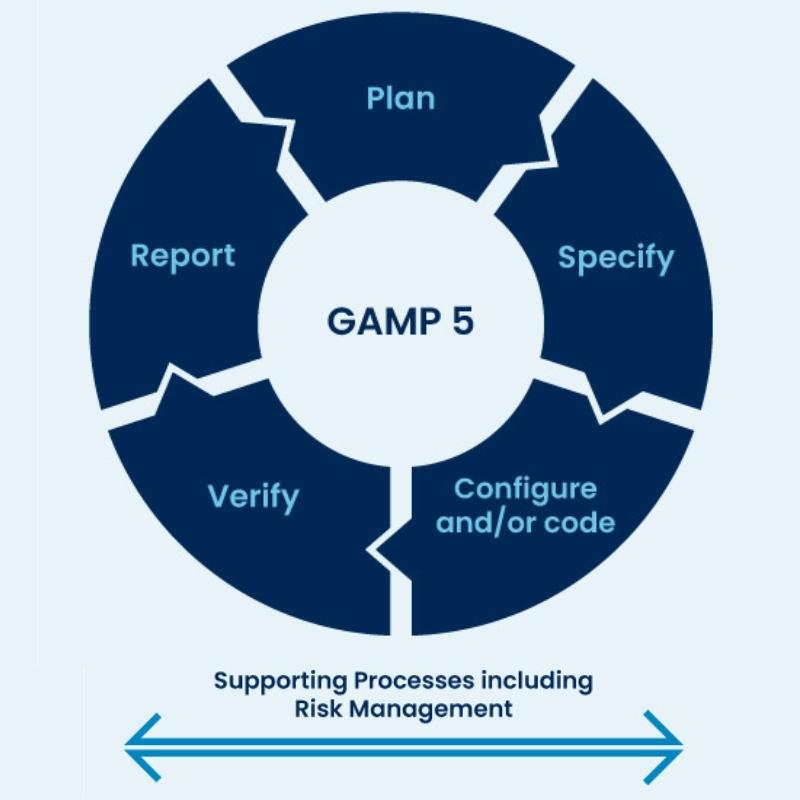
What Makes GAMP 5 Guidelines Work?
GAMP 5 guidelines use a risk-based method that’s practical and adjustable. They balance compliance with business needs, so validation isn’t overdone or skimped. Here’s how they help:
- Risk Focus: Zero in on validation where it impacts safety and quality most.
- Flexibility: Fit validation to any company, from startups to giants.
- Full Lifecycle: Cover systems from start to finish.
- Easy Records: Offer templates and steps to simplify compliance.
Take a medical device company validating a new manufacturing system. Using GAMP 5 guidelines, they focus on key parts and use standard forms, hitting compliance faster and cheaper. You get a system that’s compliant and budget-friendly.
Tying It All Together: A Full Compliance Plan
Nailing 21 CFR Part 11 isn’t just about rules. It’s blending data integrity services, Computer System Validation, and GAMP 5 guidelines into one solid strategy. Here’s how they connect:
- Start with Data
Review your data habits to find gaps and build a strong base.
- Validate Systems
Use CSV or CSA to confirm GxP systems are dependable and compliant.
- Apply GAMP 5
Use risk-based validation to streamline efforts and stay compliant long-term.
- Prep for Audits
Regular reviews and records keep you inspection-ready.
Why Team Up with Experts?
Handling 21 CFR Part 11 can be hard without know-how. That’s where business management consulting firms like Compliance Gurus shine. With over 35 years of experience, Compliance Gurus offers data integrity services, Computer System Validation, and GAMP 5 guidelines, helping all sizes of companies meet FDA, EU, and MHRA rules.
Take the Next Step Toward Compliance
Mastering 21 CFR Part 11 doesn’t need to feel impossible. With data integrity services, Computer System Validation, and GAMP 5 guidelines, you can create a compliance plan that’s strong, simple, and fits your needs. It’s more than meeting rules. It’s about safeguarding patients, products, and your reputation.
Ready to get started? Reach out to Compliance Gurus at mgregor@compliancegurus.ai or call 765.414.9332 to see how we can help you ace 21 CFR Part 11. Let’s team up to make your data and systems audit-ready and built to last.